What has Industry 4.0 got to do with your production line?
The Achema event in Frankfurt was the meeting point for the chemical and pharmaceutical industry from 11th – 15th June. The event showcased the trends towards flexible, integrated production and packaging systems, as well as the implementation of the legislative requirements for a traceable, counterfeit-proof and customer-oriented production.
All too often the pharmaceutical industry faces the need to update their production facilities. At first this can certainly be a challenge, but it also presents companies with a significant opportunity to optimise their production, implement traceability processes, protect brands and thereby increase their market share and ensure safe, customer-friendly products.
How can all this be achieved?
An up-to-date production and packaging facility is only the first step. Furthermore, the coding technology of choice needs to be suited to the respective requirements, to ensure optimum results and traceability.
Ultimately, integration with the environment’s software is crucial. Software infrastructure must enable the processing of data so that production processes are not hindered and the creation, application, control, forwarding and storage of the data runs seamlessly - and without manual interaction - in the background.
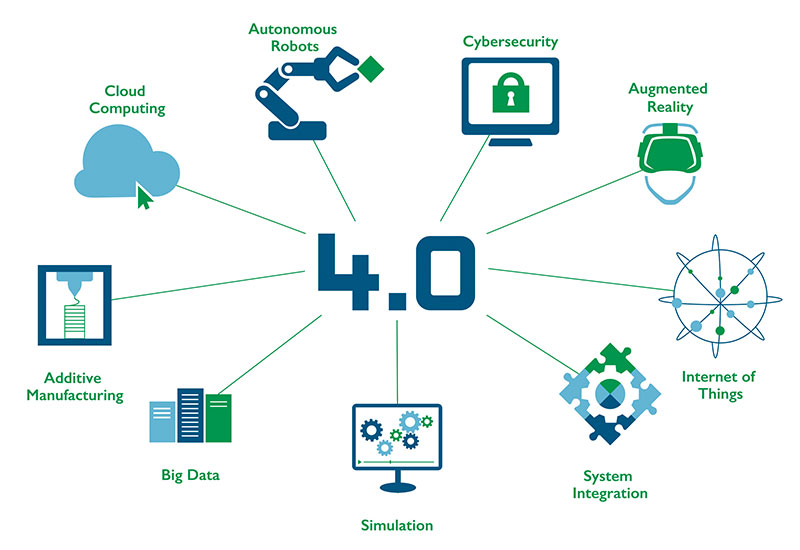
This is where the buzzword‚ Industry 4.0, comes into play: networking of multiple processes and procedures enables integrated production: the factory-wide management of production and packaging processes as well as the automated creation, testing, documentation, forwarding and saving of serialised data.
Updating production facilities is initially a troublesome task, nevertheless a critical success factor is to set-up the entire system in a structured manner and seize the opportunity to update legacy processes. The effort is worth it: Once implemented a solution based on system integration and data analysis may improve your overall equipment effectiveness (OEE), eliminate waste and gives you increased flexibility.
It is important to look for partners who can support the implementation of the complete solution – the earlier, the better. Remember, to allow six to twelve months for your solution to work well in practice.
The timeline below shows when the relevant regulations for the pharmaceutical and medical device industries come into effect. Evaluate how urgent it is for you to optimise your production.
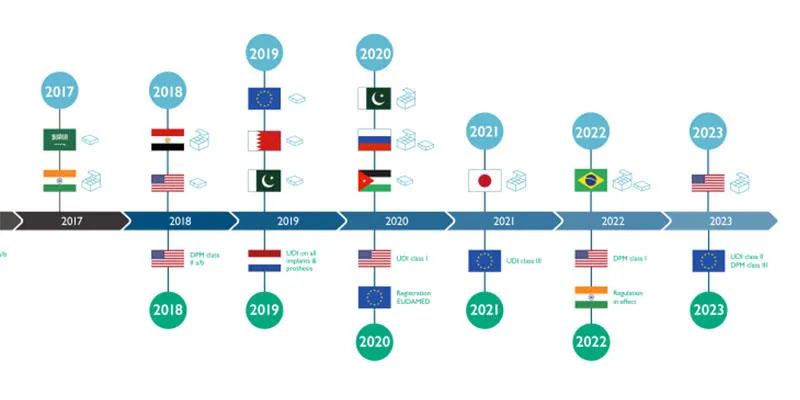
A proactive approach will mitigate any fall out and potential lost revenues through delayed adoption. An inflexible and unfocused approach may mean companies get left behind by those with a clear strategic plan and continuous improvement programme that is adapting to these latest trends widely discussed at Achema.